Meeting Program — October 2016

Keiichi Tomishige
Professor in the School of Engineering,
Tohoku University
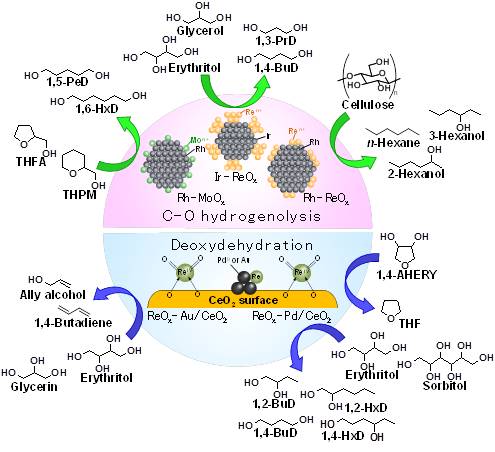
Abstract — Chemical composition of the feedstock from biomass and biomass-based building blocks has much higher oxygen contents than that from crude oil. It has been known that the target products such as monomers for the polymer synthesis have comparatively lower oxygen content, and the methodology for the decrease of the oxygen content is more and more important. One of effective methods is the utilization of the hydrogenolysis of C-O bonds in the substrates. For example, C3-C6 sugar alcohols (glycerol, erythritol, xylitol, and sorbitol) are also regarded as promising building blocks in the biomass refinery. If the selective hydrogenolysis of the target C-O bond among various kinds of the C-O bonds is possible, valuable chemicals such as diols, mono-ols, alkanes can be produced from biomass in high yield. ReOx-modified Ir metal catalyst (Ir-ReOx) is reported to be effective to the selective hydrogenolysis of polyols and cyclic ethers in water solvent. Ir-ReOx/SiO2 catalyzes the hydrogenolysis of glycerol to 1,3-propanediol. The hydrogenolysis of erythritol over the catalyst produces 1,4- and 1,3-butanediols. The selective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol also proceeds using Ir-ReOx/SiO2. In addition, the combination of Ir-ReOx/SiO2 with H-ZSM-5 gives n-alkanes and hexanols from cellulose, sugars, and sugar alcohols in high yield with the total C-O hydrogenolysis and without C-C bond dissociation and skeletal isomerization. Another interesting catalyst is ReOx-Pd/CeO2. The catalyst showed excellent performance for simultaneous hydrodeoxygenation of vicinal OH groups in various substrates. High yield (>99%), turnover frequency, and turnover number were obtained in the reaction of 1,4-anhydroerythritol to tetrahydrofuran. This catalyst is also applicable to the conversion of sugar alcohols mono-alcohols and diols are obtained in high yields from substrates with even and odd numbers of OH groups, respectively. In addition, ReOx-Au/CeO2 catalyzed the conversion of glycerol and erythritol to allyl alcohol and 1,3-butadiene in high yield (91% and 81%), respectively.
Biography — Keiichi Tomishige received his B.S., M.S. and Ph.D. from Graduate School of Science, Department of Chemistry, The University of Tokyo with Prof. Y. Iwasawa. During his Ph.D. course in 1994, he moved to Graduate School of Engineering, The University of Tokyo as a research associate and worked with Prof. K. Fujimoto. In 1998, he became a lecturer, and then he moved to Institute of Materials Science, University of Tsukuba as a lecturer in 2001. Since 2004 he has been an associate professor, Graduate School of Pure and Applied Sciences, University of Tsukuba. Since 2010, he is a professor, School of Engineering, Tohoku University.
His research interests are the development of heterogeneous catalysts for
- production of biomass-derived chemicals
- direct synthesis of organic carbonates from CO2 and alcohols
- steam reforming of biomass tar
- syngas production by natural gas reforming
He is Associate Editor of Fuel Processing Technology (2014/2-), Editorial board of Applied Catalysis A:General (2009/4-), Editorial advisory board of ACS Catalysis (2013/11-), International Advisory Board of ChemSusChem (2015/1-) and Advisory Board of Green Chemistry(2016/8-).